Librium: Drug Stories Part IV
Discovery of Chlordiazepoxide (Librium)
Leo Sternbach, a Polish American scientist working with Roche, a Swiss pharmaceutical firm was assigned the job of coming up with agents acting on nervous system. In 1950s, barbiturates had gained popularity as antianxiety agents but suffered with many side effects, addiction being the most serious. Sternbach had worked with benzoheptoxdiazines while working on his project for discovering dyes. The idea of rational drug design or rather target based drug discovery was yet to catch up. Thus, Sternbach chose to work with molecules that he was familiar with. Till then, it had come to light that drugs which where weakly basic possessed better activity on the central nervous system. Hence, he synthesised several derivatives of the compound II by reacting compound I with various secondary amines.
Benzoheptaoxadiazine derivatives
All these synthesised molecules were then sent for evaluation of biological activity. However, none showed any CNS activity. Later, it came to light that the compounds which had been thought to be benzoheptoxdiazine derivatives, were in fact, quninazoline oxides. The reassigned structure of the molecules I and II was:
Quinazoline N-oxides
The chemistry having come to light, Sternbach is
reported to have reacted in 1955, molecule III with methylamine, a primary amine in order to
achieve molecule IV.
However, due to some other impending projects, this molecule was never tested for activity and lay on the shelf at Roche laboratory was nearly two years. During a regular clean up, Sternbach’s assistant Earl Reeder came across this molecule that had not been tested for biological activity. Following a well laid out procedure that stated that no molecule was to be discarded without having undergone biological evaluation, the molecule was duly sent for the same. Sternbach and his team were in for a shock when the reports from the lab showed very promising biological activity including sedative, muscle relaxant, anxiolytic, and anticonvulsant properties. Besides, the compound was found to be safe, offering a real advantage over the current medications.
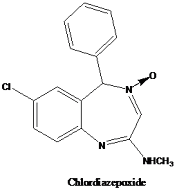
Here comes another twist in the tale-a close examination of chemistry of this molecule revealed it to be a benzodiazepine derivative rather than being a quinazoline as thought earlier. It was believed that on reaction of molecule I with methylamine, instead of a simple substitution, the molecule underwent a rearrangement leading to a ring expansion resulting in chlordiazepoxide.
Further preclinical and clinical studies confirmed the initial observations concerning its action on CNS and the molecule was introduced in the market in 1960 as Librium. This chance discovery of chlordiazepoxide, a benzodiazepine derivative, opened up the road to discovery of diazepam in 1963 and nearly 30 more derivatives during a period of two decades. However, chlordiazepoxide still remains a popularly prescribed drug for treating anxiety and is classified as a mild tranquiliser. Chlordiazepoxide is used to treat the withdrawal symptoms of acute alcoholism, to treat preoperative anxiety, and to treat anxiety over a short term period.
At the time of its discovery, mode of action of chlordiazepoxide was not known as was with the available drugs of the same category. Now it is known that it Chlordiazepoxide binds to stereospecific benzodiazepine (BZD) binding sites on GABA (A) receptor complexes at several sites within the central nervous system, including the limbic system and reticular formation. This results in an increased binding of the inhibitory neurotransmitter GABA to the GABA(A) receptor. It therefore, enhance GABA-mediated chloride influx through GABA receptor channels, causing membrane hyperpolarization. This neuro-inhibitory effect results in the observed sedative, hypnotic, anxiolytic, and muscle relaxant properties.
Chlordiazepoxide is available as 2.5mg, 5mg, 10mg,
12.5mg and 25 mg tablets and also in combination with antidepressant and
antispasmodic agents. It is a Schedule H drug and hence sold only on producing
a prescription from a registered medical practitioner.







Comments
Post a Comment